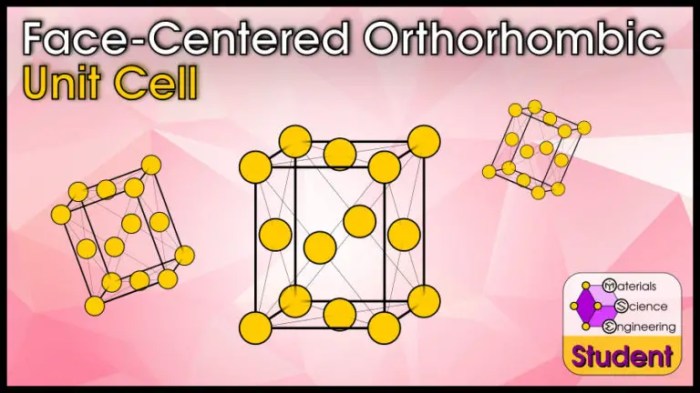
Select the unit cell for the face-centered orthorhombic crystal structure – Selecting the unit cell for the face-centered orthorhombic crystal structure is a crucial step in understanding the arrangement and properties of atoms within a crystal. This article delves into the criteria for selecting the unit cell, highlighting its importance in representing the crystal’s symmetry and determining its properties.
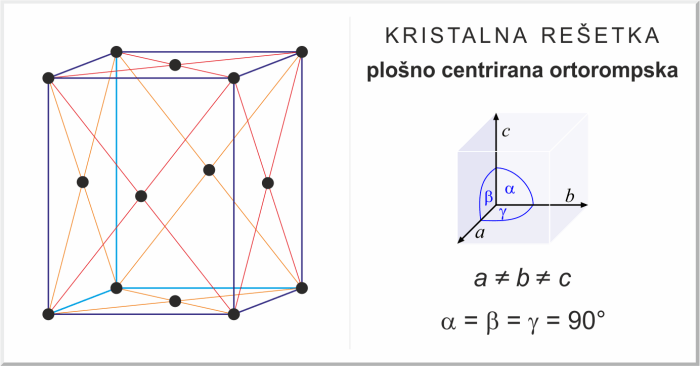
The face-centered orthorhombic crystal structure exhibits a unique arrangement of atoms, with atoms occupying the corners and centers of the faces of the unit cell. This arrangement results in a distinct set of properties, including specific dimensions, volume, and atomic packing factor.
Crystal Structure Basics
In crystallography, the concept of a unit cell is fundamental. It is the smallest repeating unit that can be used to describe the entire crystal structure. The arrangement of atoms or molecules within the unit cell determines the symmetry and properties of the crystal.
Crystals can exhibit various types of structures, including cubic, tetragonal, orthorhombic, monoclinic, triclinic, and hexagonal. Orthorhombic crystals are characterized by having three unequal axes that are perpendicular to each other.
Face-Centered Orthorhombic Crystal Structure, Select the unit cell for the face-centered orthorhombic crystal structure
The face-centered orthorhombic crystal structure is a type of orthorhombic structure where atoms are arranged at the corners and face centers of the unit cell. This results in a more compact packing of atoms compared to the primitive orthorhombic structure.
The unit cell of a face-centered orthorhombic crystal has eight atoms, with four atoms at the corners and four atoms at the centers of the six faces. The atoms at the corners are shared among eight adjacent unit cells, while the atoms at the face centers are shared among two adjacent unit cells.
Selecting the Unit Cell
When choosing the unit cell for a face-centered orthorhombic crystal, it is important to select a unit cell that represents the symmetry of the crystal. The unit cell should have the same shape and dimensions as the crystal itself.
The conventional unit cell for a face-centered orthorhombic crystal is a rectangular prism with sides that are parallel to the crystallographic axes. The dimensions of the unit cell are given by the lattice parameters a, b, and c, which are the lengths of the three axes.
Properties of the Unit Cell
The dimensions of the unit cell for a face-centered orthorhombic crystal are:
- a= length of the a-axis
- b= length of the b-axis
- c= length of the c-axis
The volume of the unit cell is given by V = abc.
The atomic packing factor (APF) for a face-centered orthorhombic crystal is 0.74, which is the ratio of the volume occupied by atoms to the volume of the unit cell.
Applications of Face-Centered Orthorhombic Crystals
Materials that exhibit a face-centered orthorhombic crystal structure include:
- Diamond
- Silicon
- Germanium
- Polyethylene
These materials are used in a wide range of applications, including:
- Electronics
- Semiconductors
- Optics
- Plastics
Detailed FAQs: Select The Unit Cell For The Face-centered Orthorhombic Crystal Structure
What is the significance of selecting the unit cell for a crystal structure?
Selecting the unit cell is crucial as it represents the smallest repeating unit of the crystal, allowing for the determination of the crystal’s symmetry and the calculation of its properties.
How does the face-centered orthorhombic crystal structure differ from other crystal structures?
The face-centered orthorhombic crystal structure is characterized by atoms occupying the corners and centers of the faces of the unit cell, resulting in a unique arrangement and set of properties.